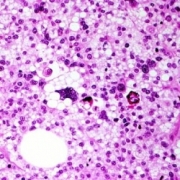
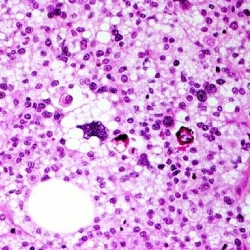
Sarcomas de partes moles de alto grau
Os Sarcomas de alto grau histológico são os tumores de maior agressividade e de pior prognóstico, devido a sua variedade histológica e raridade de casos. Geralmente os pacientes com esse diagnóstico são tratados com uma combinação de terapias mais agressivas.
O tratamento com quimioterapia neoadjuvante e adjuvante ainda é controverso, porém alguns pacientes tem se beneficiado com uso destas terapias. Ainda são necessários mais estudos com quimioterápicos, terapias alvo e imunoterapias para tratamento destas neoplasias.
O tratamento cirúrgico agressivo é mandatorio e a complementação com radioterapia tem mostrado bons resultados para diminuição dos índices de recidivas locais. A evolução clínica dos pacientes com sarcoma de alto grau dependem da histologia do tumor, margem cirúrgica da ressecção, presença de metástases a distância. Não foram observadas evidências estatísticas que relacionem a recidiva local dos sarcomas de alto grau com a recidiva local.
Referência:
Dynamic prediction of overall survival for patients with high-grade extremity soft tissue sarcoma
A.J. Rueten-Buddea,∗,1, V.M. van Praagb,1, , PERSARC studygroup (Lee M. Jeys,
Minna K. Laitinen, Rob Pollock, Will Aston, Jos A. van der Hage, PD Sander Dijkstra, Peter C. Ferguson, Anthony M. Griffin, Julie J. Willeumier, Jay S. Wunder, Emelie Styring, Florian Posch, Olga Zaikova, Katja Maretty-Kongstad, Johnny Keller, Andreas Leithner, Maria A. Smolle, Rick L. Haas), M.A.J. van de Sandeb,2, M. Fioccoa,c,2
a Mathematical Institute, Leiden University, Niels Bohrweg 1, 2333 CA, Leiden, the Netherlands
b Department of Orthopaedic Surgery, Leiden University Medical Center, Albinusdreef 2, 2333 ZA, Leiden, the Netherlands
c Department of Biomedical Data Sciences, Leiden University Medical Center, Einthovenweg 20, 2333 ZC, Leiden, the Netherlands
Evaluating the Role of Interdigitated Neoadjuvant Chemotherapy and Radiation in the Management of High-Grade Soft-Tissue Sarcoma:
The Johns Hopkins Experience
Raju R. Raval, MD, DPhil*, Deborah Frassica, MD*, Katherine Thornton, MD†, Christian Meyer, MD, PhD†, David S. Ettinger, MD†, Frank Frassica, MD‡, Kristin Weber, MD§, and Stephanie A. Terezakis, MD*
*Department of Radiation Oncology and Molecular Radiation Sciences, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD
†Department of Medical Oncology; Johns Hopkins University School of Medicine, Baltimore, MD
‡Department of Orthopaedic Surgery, Johns Hopkins University School of Medicine, Baltimore, MD
Department of Orthopaedic Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA
Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up†
12345678 P. G. Casali , N. Abecassis , S. Bauer , R. Biagini , S. Bielack , S. Bonvalot , I. Boukovinas , J. V. M. G. Bovee ,
9 10 11 ́10 12 13 14 T.Brodowicz,J.M.Broto ,A.Buonadonna ,E.DeAlava ,A.P.DeiTos ,X.G.DelMuro ,P.Dileo ,
H. Robinson , P. Rutkovski , A. A. Safwat
, P. Scho ̈ ffski , S. Sleijfer , S. Stacchiotti ,
48 49
Sundby Hall , M. Unk , F. Van Coevorden
29 51
, W. Van der Graaf , J. Whelan , E. Wardelmann
*
Zaikova
53
& J. Y. Blay
54
34 35 36 37 38
43 36 44 45 46 47
,
52
50
, on behalf of the ESMO Guidelines Committee and EURACAN
,
1Fondazione IRCCS Istituto Nazionale dei Tumori and University of Milan, Milan, Italy; 2Instituto Portugues de Oncologia de Lisboa Francisco Gentil, EPE, Lisbon, Portugal; 3University Hospital Essen, Essen, Germany; 4Department of Oncological Orthopedics, Musculoskeletal Tissue Bank, IFO, Regina Elena National Cancer Institute, Rome, Italy; 5Klinikum Stuttgart-Olgahospital, Stuttgart, Germany; 6Institut Curie, Paris, France; 7NORDIX, Athens, Greece; 8Department of Pathology, Leiden University Medical Center, Leiden, The Netherlands; 9Vienna General Hospital (AKH), Medizinische Universita ̈t Wien, Vienna, Austria; 10Hospital Universitario Virgen del Rocio-CIBERONC, Seville, Spain; 11Centro di Riferimento Oncologico di Aviano, Aviano; 12Ospedale Regionale di Treviso “S.Maria di Ca` Foncello”, Treviso, Italy; 13Integrated Unit ICO Hospitalet, HUB, Barcelona, Spain; 14Sarcoma Unit, University College London Hospitals, London, UK; 15Skane University Hospital-Lund, Lund, Sweden; 16N. N. Blokhin Russian Cancer Research Center, Moscow, Russian Federation; 17Institute of Scientific Hospital Care (IRCCS), Regina Elena National Cancer Institute, Rome; 18Pediatric Oncology Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan; 19Istituto Ortopedico Rizzoli, Bologna; 20Azienda Ospedaliera Universitaria Careggi Firenze, Florence, Italy; 21Department of Medical Oncology, Leiden University Medical Centre, Leiden, The Netherlands; 22Institut Jules Bordet, Brussels, Belgium; 23Candiolo Cancer Institute, FPO IRCCS, Candiolo, Italy; 24Turku University Hospital (Turun Yliopistollinen Keskussairaala), Turlu, Finland; 25Oxford University Hospitals NHS Foundation Trust, Oxford, UK; 26Mannheim University Medical Center, Mannheim; 27Department of Medicine III, University Hospital, Ludwig-Maximilians-University Munich, Munich, Germany; 28Helsinki University Central Hospital (HUCH), Helsinki, Finland; 29Royal Marsden Hospital, London; 30The Institute of Cancer Research, London, UK; 31University Medical Center Groningen, Groningen; 32Radboud University Medical Center, Nijmegen, The Netherlands; 33University Hospital Motol, Prague; 34Masaryk Memorial Cancer Institute, Brno, Czech Republic; 35Gustave Roussy Cancer Campus, Villejuif, France; 36Maria Skłodowska Curie Institute, Oncology Centre, Warsaw, Poland; 37Tel Aviv Sourasky Medical Center (Ichilov), Tel Aviv, Israel; 38Medical Oncology, University Hospital of Lausanne, Lausanne, Switzerland; 39Azienda Ospedaliera, Universitaria, Policlinico S Orsola-Malpighi Universita` di Bologna, Bologna; 40Azienda Ospedaliero, Universitaria Cita della Salute e della Scienza di Torino, Turin, Italy; 41Fundacio de Gestio Sanitaria de L’hospital de la SANTA CREU I Sant Pau, Barcelona, Spain; 42Helios Klinikum Berlin Buch, Berlin, Germany; 43YCRC Department of Clinical Oncology, Weston Park Hospital NHS Trust, Sheffield, UK; 44Aarhus University Hospital, Aarhus, Finland; 45Leuven Cancer Institute, Leuven, Belgium; 46Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands; 47Fondazione Istituto di Ricovero e Cura a Carattere Scientifico, Istituto Nazionale dei Tumori, Milan, Italy; 48Department of Oncology, Oslo University Hospital, The Norwegian Radium Hospital, Oslo, Norway; 49Institute of Oncology of Ljubljana, Ljubljana, Slovenia; 50Netherlands Cancer Institute Antoni van Leeuwenhoek, Amsterdam, The Netherlands; 51University College Hospital, London, UK; 52Gerhard-Domagk-Institut fu ̈r Pathologie, Universita ̈tsklinikum Mu ̈nster, Mu ̈nster, Germany; 53Oslo University Hospital, Norwegian Radium Hospital, Oslo, Norway; 54Centre Leon Bernard and UCBL1, Lyon, France
*Correspondence to: ESMO Guidelines Committee, ESMO Head Office, Via Ginevra 4, 6900 Lugano, Switzerland. E-mail: clinicalguidelines@esmo.org †Approved by the ESMO Guidelines Committee and EURACAN: December 2017.